Developmental Disabilities Grantmaking
Table of Contents

(Duke University)
Our founder, Serena Merck, had a single goal in mind when she established The John Merck Fund in 1970: to improve the lives of children with intellectual disabilities and, in the process, the lives of their parents and siblings.
For decades Serena Merck had sought the best care possible for the second of her three children, John, who was 40 years old when the Fund was founded. John had appeared to develop normally until the age of about 3, when brain damage he suffered due to meningoencephalitis of unknown cause left him with severe intellectual disability, epilepsy, and emotional disturbance until his death in 2001. In starting the Fund, Serena Merck hoped to support work toward new care models that might benefit children and families in similar straits.
For the next five decades, our developmental disabilities program employed a succession of strategies intended to “push the edge of the envelope,” as Frank Hatch, an original member of our board and its longtime chair, described it. In the 1970s, we supported work on a novel direct-care model designed to provide integral treatment of children with co-occurring neurodevelopmental disabilities and mental illness—a population that at the time was largely neglected and poorly understood. In the 1980s, we addressed a dearth of research with relevance to this population by helping four top academic centers establish junior fellowships designed to encourage talented young clinicians and basic scientists to investigate developmental disabilities.
Then, from 1990 to 2014, we refocused our program to concentrate almost exclusively on basic brain research—with fellowships tailored to attract young investigators as before, but this time in a competitive awards program not tethered to particular institutions.
Finally, after deciding in 2012 to spend out the Fund’s resources over the ensuing decade, we resolved that the program in its last chapter would once again address developmental disabilities more directly. Specifically, we supported projects aimed at bridging the divide between basic research and clinical practice, and we targeted two disorders—Fragile X syndrome and Down syndrome, the two most common genetic causes of intellectual disabilities.
While each phase of our developmental disabilities grantmaking featured a distinct strategy and focus, they all reflected an emphasis on impact, a willingness to take risks, and a desire to support outstanding individuals capable of making an enduring difference in their fields.

Among these outstanding individuals was Bradley Schlaggar, a pediatric neurologist who received a four-year John Merck Scholars research award in 2002. Schlaggar went on to serve on the Fund’s Scientific Advisory Board from 2008 to 2021, helping to oversee the shift in our program’s focus from basic research to translational research for JMF’s final decade.
“When I first applied to be a John Merck Scholar, I could not have foreseen that nearly 20 years later, I would be reflecting on how JMF’s journey would be so significantly enmeshed with my own,” said Schlaggar in 2020, two years after he had become president and CEO of the Baltimore-based Kennedy Krieger Institute, a renowned clinical care, research, and special education center for children with pediatric developmental disabilities. “I think that the legacy of The John Merck Fund is to have first seeded multiple disciplines across multiple generations with superb scientists whose work tackled fundamental mechanisms that underpin developmental disabilities, and then—boldly—to have declared that it was time to go all-in on trying to solve the most common genetic causes of neurodevelopmental disabilities.”
JMF Grants 1970-2021: Developmental Disabilities
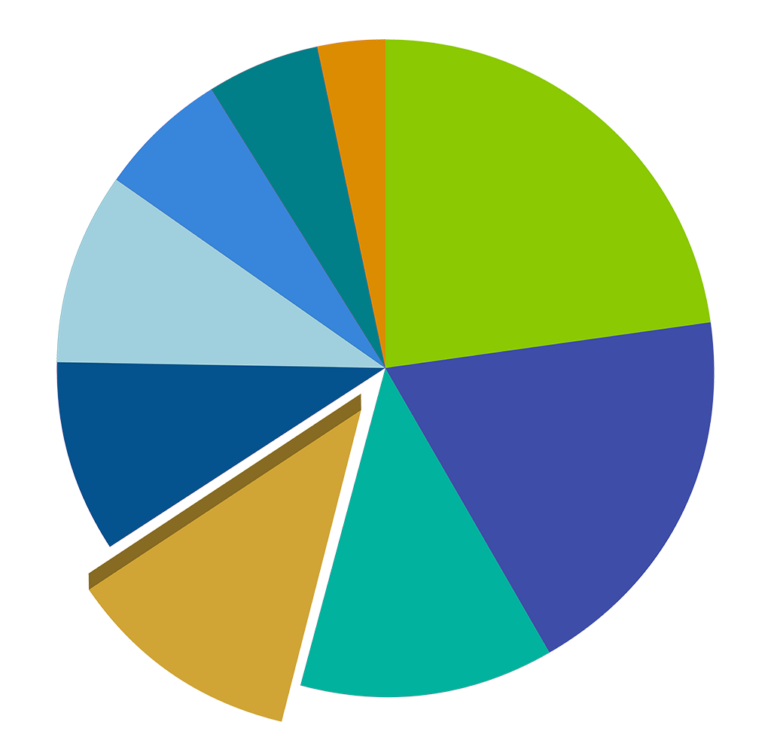
Total $ Amount
$35,319,130
Years of Program
1970-2021
# of Grants
395
Direct care model: 1970s

A catalyst for the Fund in its early years was Irene Jakab, a psychiatrist at McLean Hospital in Brookline, Massachusetts, and later a professor of psychiatry at the University of Pittsburgh, who oversaw John Merck’s care for much of his life. She was known for her luminous smile and powerful resolve to improve psychiatric care for developmentally disabled children—the latter quality perhaps symbolized by the muscle car she drove at an advanced age in Pittsburgh: a yellow Pontiac GTO.
Jakab advocated a comprehensive treatment approach for children found to have co-occurring neurodevelopmental disabilities and psychiatric disorders. It is estimated that 20% of people with intellectual disabilities have some degree of mental illness; but at the time, many of these patients were being treated exclusively for one diagnosis or the other—a dichotomy perversely reinforced by federal reimbursement regulations. Jakab proposed that a model program be piloted to provide comprehensive treatment for both, the expectation being that improved outcomes would be shown to justify the extra cost.
In 1975, such a program was launched under her direction at the University of Pittsburgh’s Western Psychiatric Institute and Clinic, using capital funding from Serena Merck and subsequent annual contributions from The John Merck Fund. Initially, the program accommodated 10 live-in patients as well as day patients, aged 5 to 10, with residential stays ranging from one to four years.
The program was pioneering, given that it was not until the early 1980s that the challenges facing those with what became known as “dual diagnosis” began attracting broad interest in the field of developmental disabilities. That interest stemmed largely from the clinical work and writings of U.S. psychologist Steven Reiss, who in 1982 introduced the now-prevalent term “diagnostic overshadowing” to explain the lack of attention paid to the mental health problems of people with intellectual disabilities. In 1983, a national association—the National Association for the Dually Diagnosed (NADD)—was formed to help healthcare professionals learn about and address the unique challenges facing people with these co-occurring conditions.
Though it met the “edge of the envelope” test, the Pittsburgh project had difficulty achieving another core aim of the Fund: proving it could be cost-effectively reproduced elsewhere. Perhaps the foremost challenge on that score was a dramatic decline nationwide in state and federal reimbursement for in-patient mental health. This trend clouded the outlook for replicability of Jakab’s model, given the emphasis on residential care at the clinic—which Jakab had hoped would eventually house 80 children.
Largely for this reason, we ended our support for the Pittsburgh program in 1982. The University of Pittsburgh, however, continued the clinic, creatively adapting it to changes in the funding environment and the field. Over the years, the clinic shifted its focus from long-term care to acute care, eventually providing 28 in-patient beds and outpatient services designed to support patients throughout their lifespan. In doing so, it maintained its commitment to a model of multi-disciplinary expert care of the kind Irene Jakab had envisioned.
Research and training: 1982-1990
Beginning in 1982, The John Merck Fund shifted its developmental disabilities strategy from direct care modeling to research fellowships and training. The goal, as Orville Schell, the Fund’s chair during those years, put it, was “to advance the basic knowledge of mental retardation in children, particularly in those who are emotionally disturbed as well, and to develop effective methods for their treatment.”
We concluded we could make the biggest difference by supporting bright young scientists in the field of developmental disabilities research. To do this, we funded fellowships for junior faculty, mainly at four universities—Stanford, Yale, Columbia, and Johns Hopkins—with $3 million over an eight-year period.

The program is credited with helping remarkable scientists establish themselves early in their careers. Among them were John Rubenstein, whose work—initially at Stanford, but mainly at the University of California, San Francisco—spotlighted the role played by genetics in the development of the forebrain, producing insights into early-childhood mental health and learning disorders.

Another of the outstanding scientists we funded was Agnes Whitaker, a Columbia University researcher who used her John Merck Fund support to conduct a pilot study of the relation between neonatal cranial ultrasound abnormalities and behavioral-emotional problems in children born prematurely. Our funding enabled her to develop a research career in which she explored long-term cognitive and psychiatric outcomes in preterm infants. Whitaker went on to direct the clinical service at Columbia University for autism and related disorders before becoming director of the Whitaker Scholar Program in Developmental Neuropsychiatry, which was established to help junior faculty develop expertise in neurodevelopmental disorders.

A third fellowship recipient, Joseph Piven, became a leader in the field of autism research. His contributions include using neuroimaging to describe a pattern of brain development in infants that predicts later diagnosis of autism, raising the possibility of presymptomatic detection and intervention.
Asked in 2020 to reflect on the JMF fellowship, Piven said a key achievement of the program was to help open developmental disabilities research for the first time to child psychiatrists. He offered himself as a case in point. After he completed a psychiatry residency at Johns Hopkins and a one-year rotation overseas, he planned to enter private practice, but then became aware of The John Merck Fund fellowship and was encouraged to apply.
“The striking thing about it was that it changed my career,” said Piven, who became a professor at the University of North Carolina and director of the Carolina Institute for Developmental Disabilities. “In those days there were no child psychiatrists doing research, [but] I got this grant to pay for three years of postdoc training and a small amount for a research assistant. If not for that, I would be out in private practice now, doing psychotherapy. To me, that’s the story. I went off to have a committed career in clinical practice and research with developmental disabilities.”
By entering a research field dominated until then by psychoanalysts, child psychiatrists such as Rubenstein, Whitaker, and Piven contributed to the understanding of problems affecting developmentally disabled children.
“Back in 1982, for instance, autism was thought to be an incredibly rare condition, one that was caused by [so-called] refrigerator mothers and their profoundly deteriorated child-maternal interactions,” Joseph Coyle, who directed the Johns Hopkins Division of Child and Adolescent Psychiatry in that period, observed in 2020. “Stanford, Hopkins, and Yale tried to address this. The people supported [by The John Merck Fund] are pioneers.”
In addition to helping talented individuals launch research careers, we also seeded training initiatives to assist more such scientists. At Yale, for instance, we supported the establishment in 1985 of a program to help next-generation researchers develop expertise in children’s developmental disorders. The Training Program in Childhood Neuropsychiatric Disorders at the Yale Child Study Center, a division of the Yale School of Medicine, attracts fellows from both clinical and basic sciences. Researchers in this program work under faculty ranging from neurobiologists and cognitive neuroscientists to geneticists and public health experts.
The interdisciplinary approach gained relevance over the years as research increasingly revealed the “interconnected and complex” nature of developmental disorders, James Leckman, the Child Study Center’s research director for over two decades, noted in a 2020 interview for this history.
Basic brain research: 1990-2014
Beginning in 1990, we steered our developmental disabilities grantmaking in a productive, though seemingly counterintuitive new direction: basic brain research. As previously, grant recipients were promising young scientists—in this case, assistant professors a few years beyond their Ph.D. degrees yet still at the outset of their independent research careers. But fellows in what came to be called the John Merck Scholars Program were not expected to address developmental disorders directly. To the contrary, the program’s competitive winnowing focused primarily on which candidates’ research held the greatest potential for breakthrough discoveries in brain science.

Harvard Medical School
This new strategy did not come without debate. A 1989 program review conducted for the Fund urged our board to build on its existing strategy and help certain academic institutions develop integrated centers of research, training, and care rather than to focus solely on basic research. “The new developments in neurobiology and genetics have excitement and promise, but it would be a grave error to believe that the problems of [dually diagnosed] children will be fully explained by metabolic errors at the molecular level,” the review said. “Human behavior is far more complex. … [E]ven where brain malfunction is present, the reaction of the family to the child and the availability to the child of remedial assistance make a very considerable difference to social outcome.”
Ultimately, however, the board embraced the vision of helping talented young investigators pursue novel brain research, largely unconstrained by subject-matter criteria. That approach had been proposed at the Fund’s invitation by Howard Hiatt, former dean of the Harvard School of Public Health, in consultation with neurophysiologist Torsten Wiesel and neuroscientist Eric Kandel, both Nobel laureates. The rationale behind the approach was that high-risk, high-reward brain research represented the best way to produce paradigm-shifting advances in the understanding and treatment of developmental disabilities, even if many projects might fail, yield unexpected results, or take years to pay off.

The need for such support was becoming acute. As a consequence of government budget cuts, the average age at which scientists could expect to see their first federal research grant had crept steadily upward—a trend prejudicial precisely to the young investigators most likely to spur innovation. And at the time, few private funders had stepped in to help fill the void.
The John Merck Fund became one of the first to do so, guided by an expert panel that was led successively by Hiatt and cognitive neuroscientist Nancy Kanwisher, and that initially included Wiesel and Kandel.

(Courtesy of University of Pennsylvania)
Over 24 years, we awarded $17.9 million in grants to young scientists, including future luminaries in the field such as 2004 John Merck Scholar Loren Frank, whose study of circuitry in the hippocampus of animals revealed brain processes of learning, remembering, and decision-making; Ted Abel (1998), who explored the mechanisms of long-term memory storage at the cellular and molecular levels; Elizabeth Brannon (2003), a cognitive neuroscientist whose work illuminated the numerical abilities of human infants and nonhuman primates; and Linda Buck (1993), whose exploration of the mammalian olfactory system won her a Nobel Prize in 2004.

Some of the funded work did bear directly on developmental disorders—for example, the projects of Pawan Sinha (2003), Bradley Schlaggar (2002), B.J. Casey (1997), and John Rubenstein, who returned as a Fund grantee under the Scholars Program in 1992. In brief:
- Sinha used his Fund-supported work on how the brain recognizes objects through visual experience as a springboard for exploring the possible role of perception deficits in autism;
- Schlaggar analyzed brain scans to compare the functional neuroanatomy of cognitive development in children with and without developmental disorders;
- Casey employed functional MRI to posit that disruption in the normal development of the basal ganglia region of the brain can predispose children to attention deficit hyperactivity disorder; and
- Rubenstein continued exploring genetic mechanisms in brain development as a foundation for understanding developmental disorders.

(Pawan Sinha)
In addition, dozens of the funded projects contributed to branches of neuroscience that are key to understanding developmental disabilities, including synapse formation and synaptic plasticity; learning, memory, and synaptic plasticity; perception cognition and behavior; neurogenesis and pattern formation; and genetics and early development.
During these years, we also worked outside the context of the Scholars Program to broaden interest and expertise in the field of developmental disabilities. From 1994 to 2002, for instance, we provided just over $1 million to train young social scientists in developmental disorders, targeting four universities: Brandeis, Northeastern, the University of Wisconsin–Madison, and Vanderbilt. And from 2003 to 2010, we sponsored the John Merck Fund Summer Institute, a course run by B.J. Casey for graduate students and postdoctoral fellows.

(Courtesy of B.J. Casey)
Participants learned basic principles of normal and atypical behavioral and brain development in the course, which initially was held at Cold Spring Harbor Laboratory before shifting to Princeton University and subsequently to Cornell University and Weill Cornell Medical College. Meanwhile, during 1997–2007 the Fund underwrote the Serena Merck Award, a prize given annually by the National Association for the Dually Diagnosed to an individual providing exemplary day-to-day care for mentally and emotionally challenged children.
Still, the vast bulk of our support for developmental disabilities work during the period went toward what might be described as the long game—unfettered basic brain research. This emphasis, as Frank Hatch put it not long after the Scholars Program’s 10th anniversary, sprang from the view that basic research is necessarily wide ranging, “like a dragger which scoops up a lot of other things from the sea bottom besides scallops.” In a memo to the board, Hatch cited our expert panel’s view that “we won’t get the results we want by limiting our Scholars to our field of interest,” adding, “This is a fact of research life we have to accept.”

An independent review of the Scholars Program six years later would offer a different perspective, observing that novel research avenues and a recent “explosion” of insights into brain development offered the Fund compelling new opportunities to address developmental disorders more directly.
The review, conducted in 2007 by neuroscientist Gerald Fischbach and cognitive neuroscientist Charles Nelson, also recommended abandoning “dual diagnosis” as the ostensible program focus. They noted that while the term was valid in a broad sense, it no longer reflected the increasingly complex picture of cognitive and emotional disorders then emerging in the field.
At the same time, however, Fischbach and Nelson concluded that the Fund’s decadeslong commitment had borne extraordinary fruit. “By all accounts, the Scholars Program has been a resounding success,” they wrote. “It is now among the most visible and sought-after awards for young neuroscience investigators. The selection committee has chosen wisely, as alumni of the program have assumed leadership roles in the field of developmental disorders throughout the land.”
Translational research: 2012-2021
The developmental disabilities program took a final strategic turn in 2012, after the Fund had come under a new generation of leadership and decided to spend out its assets over the next 10 years. Weighing various alternatives, the board chose to concentrate on translational research, with grants focused on Fragile X syndrome and Down syndrome, both of which were deemed ripe for treatment advances.
The new course of action reflected the view, expressed in Fischbach and Nelson’s report, that research breakthroughs had created unprecedented opportunities to bring fundamental science to bear on specific disabilities. It also was rooted in the board’s desire to focus the program’s final years on projects that might lead more directly to treatment advances for affected children and their families.

Guided by a scientific advisory board headed by Marsha Mailick, then director of the University of Wisconsin’s Waisman Center, we awarded $8.4 million in translational research grants over 10 years. In addition, we made six grants totaling $6.4 million to help launch a statewide newborn-screening initiative in North Carolina, an effort aimed at expanding research opportunities and spurring early diagnosis and treatment of inherited developmental disorders.
Under the new strategy, grants were no longer earmarked solely for basic scientists at the outset of their careers. Instead, the goal was to “bridge basic and clinical science, [and to] more rapidly translate findings into treatment settings and promulgate best clinical practices.” Funded projects ran the research gamut, ranging from ambitious bids for game-changing impact, to surer-bet attempts to advance knowledge in the field, to highly targeted work aimed at immediate diagnostic and clinical improvement.
Each project, though, addressed one of the two target conditions—Fragile X syndrome, an inherited disorder that disrupts production of a protein needed for normal brain development, and Down syndrome, in which a surplus chromosome affects development of both the body and brain.
“Though advances in the past had yielded extraordinary knowledge and insights about brain development and dysfunction, there was what I call a ‘valley of death,’ or chasm, between these insights and the lives of people with developmental disabilities and their families,” Mailick said in a 2020 interview. “Bridging this chasm motivated the final chapter of the JMF developmental disabilities program. Translational research, if successful, could have both immediate impact on families [and] long-range impacts for understanding basic brain function and dysfunction.”
At the basic-science end of the spectrum, developmental biologist Jeanne Lawrence explored the use of a naturally occurring gene to “silence” the extra chromosome that causes Down syndrome, a strategy that she showed was successful in patient-derived pluripotent stem cells. This proof of principle in vitro suggested the potential to develop a breakthrough chromosomal therapy. We supported an ongoing effort by Lawrence to test the strategy in a mouse model of Down syndrome as well as work by Lawrence and her team showing successful trisomy silencing in human neural cells in vitro.

Meanwhile, University of Wisconsin neuroscientists Xinyu Zhao and Anita Bhattacharyya joined clinician Craig Erickson of Cincinnati Children’s Hospital to create stem cells from the blood of Fragile X patients in order to study the mechanisms underlying “brain neuron hyperexcitability,” a type of change in brain electrical activities associated with behavioral difficulties. Neuron hyperexcitability particularly affects about one-third of boys with Fragile X, an estimated 40%–70% of whom have severe behavioral problems.
The stem cell lines were made available for use in the longer term, allowing researchers to explore why some patients exhibit hyperexcitability while others do not, and to screen drugs targeting diverse subpopulations of Fragile X symptoms. Because the stem cells survive indefinitely in special freezers, they could serve as a resource for therapeutic discovery by researchers worldwide.
Other funded projects sought more immediate improvements in the lives of those with developmental disabilities, and—by extension—the lives of their family members. Support for such work meant taking risks. Results could not be predicted, no matter how well led or potentially impactful a project might be. For instance, Erickson, a leading expert in Fragile X, conducted a research project to see whether young people with that syndrome might benefit from Acamprosate, a drug used to treat alcoholism, but the tests did not show significant impact.
Yet a trial of a telehealth behavioral intervention designed to help boys with Fragile X showed impressive results. The telehealth intervention, developed by Scott Hall of Stanford University, involved remote engagement with parents and caregivers of 30 boys whose age averaged 6.8 years. Two-thirds of the 24 boys whose families completed the treatment responded positively as defined by an improvement of at least 25% over the course of the 12-week study. In a 2019 report to the Fund, Hall wrote: “[T]he results of our clinical trial hold vast promise to make real and meaningful differences in the lives of boys with [Fragile X] with severe disruptive behaviors and [in the lives of] their families.”
Arguably the most complex project supported by our developmental disabilities program was Early Check, the statewide, voluntary newborn-screening initiative in North Carolina.

The program, led by Don Bailey, an early-childhood development expert at RTI International (RTI), targeted Fragile X and other rare disorders, which often go undiagnosed for years because they do not meet the strict criteria for inclusion in standard newborn screening. Project organizers, aiming to create procedures and practices that could be replicated throughout the United States and the world, forged a partnership with the North Carolina State Laboratory of Public Health and created a recruitment model to reach out to every mother in the state soon after she gave birth.
Participating parents received extra screening, free-of-charge, of their baby’s standard newborn-screening blood spot to determine whether the infant had one of the targeted disorders, which as of 2020 included Fragile X, Duchenne muscular dystrophy, and spinal muscular atrophy. When one of the disorders was detected, the parents received—also at no charge—genetic counseling, a review of the baby’s development, and help finding doctors and support services to enable early intervention.
Early Check exemplified how early philanthropic support can lead to broad multistakeholder involvement—collaboration of the kind the Fund tried to encourage over the years in all its program areas. From an exploratory meeting in a Washington, D.C., conference room, the program blossomed into a partnership involving RTI, Duke University, Wake Forest School of Medicine, the University of North Carolina at Chapel Hill, and the North Carolina State Laboratory of Public Health.
Its funding support began with seed contributions from The John Merck Fund and RTI, then grew to include financial and in-kind backing from the National Institutes of Health, the North Carolina Translational and Clinical Sciences Institute, Cure SMA, the Muscular Dystrophy Association, and two companies—Asuragen and Sarepta Therapeutics.
“Without the support of The John Merck Fund, Early Check would not exist—or certainly not in its current form,” Bailey, the program’s founder, said in 2020. “JMF provided critical funding to add Fragile X to the Early Check screening panel, develop and pilot an early intervention program for infants with Fragile X syndrome, and develop and implement a model for statewide telegenetic counseling. Less obvious, but perhaps more importantly, JMF board members made suggestions that fundamentally influenced the nature of Early Check and challenged the Early Check team to think bigger and more broadly.”
